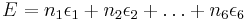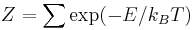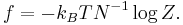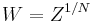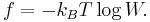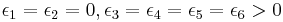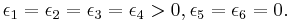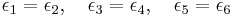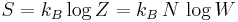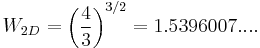Ice-type model
In statistical mechanics, the ice-type models or six-vertex models are a family of vertex models for crystal lattices with hydrogen bonds. The first such model was introduced by Linus Pauling in 1935 to account for the residual entropy of water ice[1]. Variants have been proposed as models of certain ferroelectric[2] and antiferroelectric[3] crystals.
In 1967, Elliott H. Lieb found the exact solution to a two-dimensional ice model known as "square ice"[4]. The exact solution in three dimensions is only known for a special "frozen" state[5].
Contents |
Description
An ice-type model is a lattice model defined on a lattice of coordination number 4 - that is, each vertex of the lattice is connected by an edge to four "nearest neighbours". A state of the model consists of an arrow on each edge of the lattice, such that the number of arrows pointing inwards at each vertex is 2. This restriction on the arrow configurations is known as the ice rule.
For two-dimensional models, the lattice is taken to be the square lattice. For more realistic models, one can use a three-dimensional lattice appropriate to the material being considered; for example, the hexagonal ice lattice is used to analyse ice.
At any vertex, there are six configurations of the arrows which satisfy the ice rule (justifying the name "six-vertex model"). The valid configurations for the (two-dimensional) square lattice are the following:
The energy of a state is understood to be a function of the configurations at each vertex. For square lattices, one assumes that the total energy 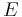 is given by
is given by
for some constants 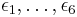 , where
, where 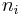 here denotes the number of vertices with the
here denotes the number of vertices with the 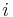 th configuration from the above figure. The value
th configuration from the above figure. The value 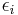 is the energy associated with vertex configuration number
is the energy associated with vertex configuration number 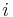 .
.
One aims to calculate the partition function 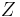 of an ice-type model, which is given by the formula
of an ice-type model, which is given by the formula
where the sum is taken over all states of the model, and where 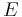 is the energy of the state,
is the energy of the state, 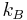 is Boltzmann's constant, and
is Boltzmann's constant, and 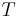 is the system's temperature.
is the system's temperature.
Typically, one is interested in the thermodynamic limit in which the number 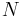 of vertices approaches infinity. In that case, one instead evaluates the free energy per vertex
of vertices approaches infinity. In that case, one instead evaluates the free energy per vertex 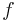 in the limit as
in the limit as 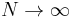 , where
, where 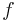 is given by
is given by
Equivalently, one evaluates the partition function per vertex 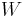 in the thermodynamic limit, where
in the thermodynamic limit, where
The values 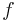 and
and 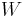 are related by
are related by
Physical justification
Several real crystals with hydrogen bonds satisfy the ice model, including ice[1] and potassium dihydrogen phosphate KH2PO4[2] (KDP). Indeed, such crystals motivated the study of ice-type models.
In ice, each oxygen atom is connected by a bond to four other oxygens, and each bond contains one hydrogen atom between the terminal oxygens. The hydrogen occupies one of two symmetrically located positions, neither of which is in the middle of the bond. Pauling argued[1] that the allowed configuration of hydrogen atoms is such that there are always exactly two hydrogens close to each oxygen, thus making the local environment imitate that of a water molecule, H2O. Thus, if we take the oxygen atoms as the lattice vertices and the hydrogen bonds as the lattice edges, and if we draw an arrow on a bond which points to the side of the bond on which the hydrogen atom sits, then ice satisfies the ice model.
Similar reasoning applies to show that KDP also satisfies the ice model.
Specific choices of vertex energies
On the square lattice, the energies 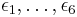 associated with vertex configurations 1-6 determine the relative probabilities of states, and thus can influence the macroscopic behaviour of the system. The following are common choices for these vertex energies.
associated with vertex configurations 1-6 determine the relative probabilities of states, and thus can influence the macroscopic behaviour of the system. The following are common choices for these vertex energies.
The ice model
When modelling ice, one takes  , as all permissible vertex configurations are understood to be equally likely. In this case, the partition function
, as all permissible vertex configurations are understood to be equally likely. In this case, the partition function 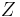 equals the total number of valid states. This model is known as the ice model (as opposed to an ice-type model).
equals the total number of valid states. This model is known as the ice model (as opposed to an ice-type model).
The KDP model of a ferroelectric
Slater[2] argued that KDP could be represented by an ice-type model with energies
For this model (called the KDP model), the most likely state (the least-energy state) has all horizontal arrows pointing in the same direction, and likewise for all vertical arrows. Such a state is a ferroelectric state, in which all hydrogen atoms have a preference for one fixed side of their bonds.
Rys 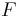 model of an antiferroelectric
model of an antiferroelectric
The Rys 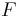 model[3] is obtained by setting
model[3] is obtained by setting
The least-energy state for this model is dominated by vertex configurations 5 and 6. For such a state, adjacent horizontal bonds necessarily have arrows in opposite directions and similarly for vertical bonds, so this state is an antiferroelectric state.
The zero field assumption
If there is no ambient electric field, then the total energy of a state should remain unchanged under a charge reversal, i.e. under flipping all arrows. Thus one may assume without loss of generality that
This assumption is known as the zero field assumption, and holds for the ice model, the KDP model, and the Rys F model.
History
The ice rule was introduced by Linus Pauling in 1935 to account for the residual entropy of ice that had been measured by William F. Giauque and E. L. Stout[6]. The residual entropy, 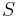 , of ice is given by the formula
, of ice is given by the formula
where 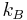 is Boltzmann's constant,
is Boltzmann's constant, 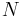 is the number of oxygen atoms in the piece of ice, which is always taken to be large (the thermodynamic limit) and
is the number of oxygen atoms in the piece of ice, which is always taken to be large (the thermodynamic limit) and 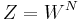 is the number of configurations of the hydrogen atoms according to Pauling's ice rule. Without the ice rule we would have
is the number of configurations of the hydrogen atoms according to Pauling's ice rule. Without the ice rule we would have 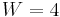 since the number of hydrogen atoms is
since the number of hydrogen atoms is 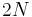 and each hydrogen has two possible locations. Pauling estimated that the ice rule reduces this to
and each hydrogen has two possible locations. Pauling estimated that the ice rule reduces this to 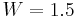 , a number that would agree extremely well with the Giauque-Stout measurement of
, a number that would agree extremely well with the Giauque-Stout measurement of 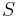 . It can be said that Pauling's calculation of
. It can be said that Pauling's calculation of 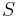 for ice is one of the simplest, yet most accurate applications of statistical mechanics to real substances ever made. The question that remained was whether, given the model, Pauling's calculation of
for ice is one of the simplest, yet most accurate applications of statistical mechanics to real substances ever made. The question that remained was whether, given the model, Pauling's calculation of 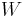 , which was very approximate, would be sustained by a rigorous calculation. This became a significant problem in combinatorics.
, which was very approximate, would be sustained by a rigorous calculation. This became a significant problem in combinatorics.
Both the three-dimensional and two-dimensional models were computed numerically by John F. Nagle in 1966[7] who found that 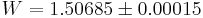 in three-dimensions and
in three-dimensions and 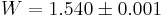 in two-dimensions. Both are amazingly close to Pauling's rough calculation, 1.5.
in two-dimensions. Both are amazingly close to Pauling's rough calculation, 1.5.
In 1967, Lieb found the exact solution of three two-dimensional ice-type models: the ice model[4], the Rys 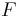 model,[8] and the KDP model[9]. The solution for the ice model gave the exact value of
model,[8] and the KDP model[9]. The solution for the ice model gave the exact value of 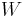 in two-dimensions as
in two-dimensions as
which is known as Lieb's square ice constant.
Later in 1967, Bill Sutherland generalised Lieb's solution of the three specific ice-type models to a general exact solution for square-lattice ice-type models satisfying the zero field assumption[10].
Still later in 1967, C. P. Yang[11] generalised Sutherland's solution to an exact solution for square-lattice ice-type models in a horizontal electric field.
In 1969, John Nagle derived the exact solution for a three-dimensional version of the KDP model, for a specific range of temperatures[5]. For such temperatures, the model is "frozen" in the sense that (in the thermodynamic limit) the energy per vertex and entropy per vertex are both zero. This is the only known exact solution for a three-dimensional ice-type model.
Relation to eight-vertex model
The eight-vertex model, which has also been exactly solved, is a generalisation of the (square-lattice) six-vertex model: to recover the six-vertex model from the eight-vertex model, set the energies for vertex configurations 7 and 8 to infinity. Six-vertex models have been solved in some cases for which the eight-vertex model has not; for example, Nagle's solution for the three-dimensional KDP model[5] and Yang's solution of the six-vertex model in a horizontal field[11].
Boundary conditions
This ice model provide an important 'counterexample' in statistical mechanics: the bulk free energy in the thermodynamic limit depends on boundary conditions[12]. The model was analytically solved for periodic boundary conditions, anti-periodic, ferromagnetic and domain wall boundary conditions. Six vertex model with domain wall boundary conditions on a square lattice has specific significance for algebraic combinatorics, it helps to enumerate Alternating sign matrix. In this case the partition function can be represented as a determinant of a matrix (dimension of the matrix is equal to the size of the lattice), but in the other cases the enumeration of 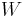 does not come out in such a simple closed form.
does not come out in such a simple closed form.
Domain wall gives the smallest 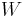 . Clearly, the largest
. Clearly, the largest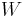 is given by free boundary conditions (no constraint at all on the configurations on the boundary), but the same
is given by free boundary conditions (no constraint at all on the configurations on the boundary), but the same 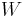 occurs, in the thermodynamic limit, for periodic boundary conditions[13], as used originally to derive
occurs, in the thermodynamic limit, for periodic boundary conditions[13], as used originally to derive 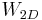 .
.
3-colorings of a lattice
The number of states of an ice type model on the internal edges of a finite simply connected union of squares of a lattice is equal to one third of the number of ways to 3-color the squares, with no two adjacent squares having the same color. This correspondence between states is due to Andrew Lenard and is given as follows. If a square has color i = 0, 1, or 2, then the arrow on the edge to an adjacent square goes left or right (according to an observer in the square) depending on whether the color in the adjacent square is i+1 or i−1 mod 3. There are 3 possible ways to color a fixed initial square, and once this initial color is chosen this gives a 1:1 correspondence between colorings and arrangements of arrows satisfying the ice-type condition.
See also
References
Notes
- ^ a b c L. Pauling, Jour. American Chem. Soc. 57, p.2680 (1935),
- ^ a b c J. C. Slater, Jour. Chemi. Phys. 9, p.16 (1941)
- ^ a b F. Rys, Helv. Phys. Acta. 36, p.537 (1963)
- ^ a b E. H. Lieb, Physical Review 162, 162-172 (1967)
- ^ a b c J. F. Nagle, Communications in Mathematical Physics, 13, 62-7 (1969)
- ^ W. F. Giauque and E. L. Stout, Physical Review 43, p.81 (1933)
- ^ J. F. Nagle, Jour. Mathematical Physics 7, p.1484 (1966).
- ^ E. H. Lieb, Physical Review Letters 18, 692-694 (1967)
- ^ E.H. Lieb, Physical Review Letters 19, 108-10 (1967)
- ^ B. Sutherland, Physical Review Letters 19, 103-4 (1967)
- ^ a b C. P. Yang, Physical Review Letters 19, 586-8 (1967)
- ^ V. Korepin, P. Zinn-Justin, Thermodynamic limit of the Six-Vertex Model with Domain Wall Boundary Conditions, Jour. Phys. A 33 No. 40 (2000), 7053, see also [1]
- ^ H. J. Brascamp and H. Kunz, Jour. Mathematical Physics 14, p.1927 (1973); doi:10.1063/1.1666271
Further References
- Lieb, E.H.; Wu, F.Y. (1972), "Two Dimensional Ferroelectric Models", in C. Domb; M. S. Green; J. Lebowitz, Phase Transitions and Critical Phenomena, 1, New York: Academic Press, pp. 331–490
- Baxter, Rodney J. (1982), Exactly solved models in statistical mechanics, London: Academic Press Inc. [Harcourt Brace Jovanovich Publishers], ISBN 978-0-12-083180-7, MR690578, http://tpsrv.anu.edu.au/Members/baxter/book